IDRs in Fungi
Intrinsically Disordered Regions (IDRs) are polypeptide segments characterized by their structural flexibility. These regions often lack hydrophobic residues, which is the primary drivers of protein folding, and are instead enriched with polar and charged residues, which enhance their solubility and interaction with the aqueous environment. This distinct amino acid composition is leveraged by computational tools, such as IUPred2, to predict IDRs directly from primary peptide sequences.
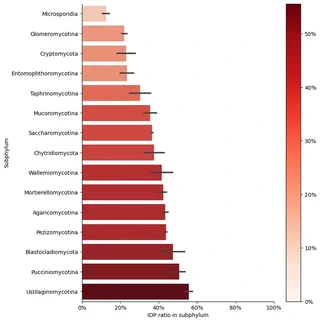
In this project, we analyzed peptide sequences from 1,014 fungal species curated by Ensembl, identifying over 8 million predicted IDRs using IUPred2. Our analysis of the IDP/peptide ratio revealed significant variation across taxonomic groups. For example, Microsporidia exhibited the lowest prevalence, with only 12.5% of their peptides containing IDRs. In contrast, more than half of the proteome in Ustilaginomycotina was found to contain disordered regions.
To further explore these trends, we investigated the relationship between the IDP/peptide ratio and genome size. We have provided an interactive scatterplot to visualize these results, categorized by subphylum.
